78 sunscreen and after-sun care products contained benzene, an industrial chemical known to cause cancer and other potentially serious health risks
See Benzene free sunscreen on Amazon
Valisure LLC has tested and detected high levels of benzene, a known human carcinogen, in several brands and batches of sunscreen, which are considered drug products by the Food and Drug Administration (FDA), as well as in after-sun care products, which are generally regulated by FDA as cosmetics.
Benzene is known to cause cancer in humans according to the U.S. Centers for Disease Control and Prevention, the U.S. Department of Health and Human Services, the World Health Organization, and other regulatory agencies. The National Institute for Occupational Safety and Health (NIOSH) defines benzene as a carcinogen and lists “inhalation, skin absorption, ingestion, skin and/or eye contact” as exposure routes. 27% of samples tested by Valisure contained detectable benzene and some batches contained up to three times the conditionally restricted FDA concentration limit of 2 parts per million (ppm).

Valisure is asking for a recall of the contaminated batches and requesting FDA better define limits for benzene contamination in drug and cosmetic products. It is important to note that not all sunscreen products contain benzene and that uncontaminated products are available, should continue to be used, and are important for protecting against potentially harmful solar radiation.
Valisure’s FDA Citizen Petition: Complete Valisure’s FDA Citizen Petition on Sun Care Products (All attachments and other resources linked below.)
Valisure is accepting sunscreen products for analysisa at no cost: Sunscreen Crowdsourcing Study Link
ConsumerLab.com has published a reviewb for its members of the Valisure data suggesting products to avoid and which might be safest with regard to the benzene results. Link to review
FDA currently recognizes the serious danger of benzene and lists it as a “Class 1 solvent” that “should not be employed in the manufacture of drug substances, excipients, and drug products because of their unacceptable toxicity…However, if their use is unavoidable in order to produce a drug product with a significant therapeutic advance, then their levels should be restricted” and benzene is restricted to 2 ppm for these special circumstances.
Being that many of the tested sunscreen and after-sun care products did not contain detectable levels of benzene, it does not appear that benzene use is unavoidable for their manufacture and considering the long history of widespread use of these products, it also does not appear that they currently constitute a significant therapeutic advance; therefore, any significant detection of benzene should be deemed unacceptable.
The toxicity of benzene in humans has been well established for over 120 years. The hematotoxicity of benzene has been described as early as 1897. A study from 1939 on benzene stated that “exposure over a long period of time to any concentration of benzene greater than zero is not safe,” which is a comment reiterated in a 2010 review of benzene research specifically stating “There is probably no safe level of exposure to benzene, and all exposures constitute some risk in a linear, if not supralinear, and additive fashion.”
Benzene is specifically associated with blood cancers such as leukemia, making absorption through the skin particularly concerning as there have been multiple studies by FDA researchers showing that chemicals in sunscreen products are found in the blood at high levels after application to the skin.
“Benzene is one of the most studied and concerning human carcinogens known to science. Its association with forming blood cancers in humans has been shown in numerous studies at trace levels of parts per million and below. The presence of this known human carcinogen in products widely recommended for the prevention of skin cancer and that are regularly used by adults and children is very troubling,” said David Light, Founder and CEO of Valisure.
The FDA has clearly determined that benzene should not be used in standard pharmaceutical production at all because of its unacceptable toxicity; however, there currently does not exist any established exposure limit for benzene. The 2ppm concentration limit only applies in special circumstances, which do not include sunscreen manufacturing. Therefore, in addition to recalls, Valisure is also petitioning the FDA to create a concentration limit for standard drug products, including sunscreen, and to also set a daily exposure limit.
Public concern regarding contamination of major drug products has significantly increased following a string of medication recalls over the past three years. Most of these recalls of drugs like valsartan, ranitidine, and metformin have been due to the “nitrosamine” class of carcinogens, specifically N-Nitrosodimethylamine (NDMA), which is a Group 2 “probable human carcinogen.”
Although NDMA has not been directly linked to cancer in humans, it has both a concentration limit, which ranges between 0.3 – 3.0 ppm for -sartan medications, and a total daily intake limit of 96 nanograms (ng), which is kept constant for all drug products
Table of limits set for NDMA and benzene in drug products:
| NDMA – Standard Drugs | Benzene – Special Circumstances** | Benzene – Standard Drugs | |
|---|---|---|---|
| Concentration limit (parts per million) | 0.3 – 3.0 ppm* | 2.0 ppm | Not specified*** |
| Daily limit (nanograms) | 96 ng | Not specified | Not specified*** |
* Dependent on the drug product. These numbers refer to guidance specifically for -sartan medications.
** A drug product constituting a “significant therapeutic advance” where the use of benzene for manufacturing is “unavoidable,” or FDA emergency guidance for hand sanitizer during the COVID-19 pandemic.
*** FDA states that benzene should not be employed in the manufacture of drug substances because of its unacceptable toxicity but does not specifically define limits.
Under “special circumstances,” where benzene is unavoidable for the manufacture of a drug with a “significant therapeutic advance,” the 2 ppm limit is similar to that of NDMA. If a similar daily exposure limit is set for benzene of 96 ng, then using the most contaminated sunscreen product Valisure identified of 6.26 ppm equates to approximately 695,800 ng of benzene or 7,248 times the NDMA limit.
“There is not a safe level of benzene that can exist in sunscreen products,” stated Dr. Christopher Bunick, MD, PhD, Associate Professor of Dermatology at Yale University. “Even benzene at 0.1 ppm in a sunscreen could expose people to excessively high nanogram amounts of benzene.”
Further support for this concern is seen in a 2019 study on sunscreen ingredients where FDA researchers stated, “Understanding the extent of systemic exposure of [sunscreen] products is important, as even a low percentage of systemic absorption could represent a significant systemic exposure.” This study also found that significant amounts of the ingredients in sunscreens are absorbed through the skin and were detected in the blood at high levels.
Additionally, a study by Health Canada’s Bureau of Chemical Hazards has shown that the application of sunscreen specifically increases the absorption rate of benzene through the skin. Absorption through skin and into the blood is particularly concerning regarding benzene, which is associated specifically with blood cancers.
Decades of research has shown that regular exposure to benzene at concentrations of 1 ppm, or potentially even lower, have been clearly associated with the development of cancers of blood tissues, such as leukemia. This research has largely focused on exposure through inhalation, though absorption of benzene through skin has also been well established. The National Institute for Occupational Safety and Health (NIOSH) recommends protective equipment be worn by workers expecting to be exposed to benzene at concentrations of 0.1 ppm and defines “skin absorption” as an exposure route.
Valisure’s March 24, 2021 Citizen Petition on benzene contamination in hand sanitizer, and the recent recalls of contaminated hand sanitizer products due to the presence of benzene, further underscores the necessity to better regulate benzene and its apparent prevalence in the drug and consumer product supply chains.
Beyond the significant concern for public health, there is also evidence that both sunscreen products and benzene pose a serious risk to the environment, marine ecosystems, and United States waterways. The National Oceanic and Atmospheric Administration (“NOAA”) has published reports and infographics intended to educate consumers regarding the potential for sunscreen products to threaten corals and other marine life.
Additionally, scientific papers published by NOAA have shown that benzene can be absorbed by fish and short-term exposure (48 hours) to concentrations of benzene at parts per billion levels can significantly reduce survival of certain fish eggs.
Furthermore, NOAA has proposed that the use of sunscreen followed by swimming or showering may cause sunscreen chemicals to wash off and enter waterways, an area of significant concern to the Environmental Protection Agency (“EPA”) which extensively regulates benzene. Strict EPA regulations on benzene are detailed in a report authored by the Agency for Toxic Substances and Disease Registry (“ATSDR”) which stated that, “EPA has set 5 ppb [equivalent to 0.005 ppm] as the maximum permissible level of benzene in drinking water. EPA has set a goal of 0 ppb for benzene in drinking water and in water such as rivers and lakes because benzene can cause leukemia.”
Valisure’s Findings
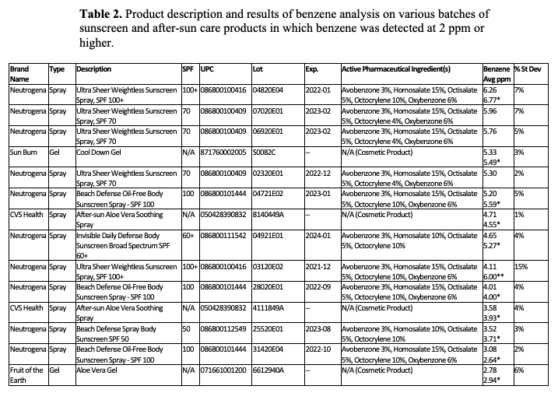
Valisure analyzed 294 unique batches from 69 different companies. Significant variability from batch to batch was observed, even within a single company. Fourteen lots of sunscreen and after-sun care products from four different brands contained between 2.78 – 6.26 ppm of benzene; 26 lots from eight brands contained detectable benzene between 0.11 – 1.99 ppm; and 38 lots from 17 brands contained detectable benzene at < 0.1 ppm. Benzene was not detected in an additional 217 batches of sunscreen from 66 different brands through initial analysis of at least one sample. Benzene contamination was detected in sprays, gels, and lotions with both chemical and mineral-based formulations.
Benzene is a colorless or light-yellow liquid chemical at room temperature. It has been used primarily as a solvent in the chemical and pharmaceutical industries and is well known to cause cancer in humans. Trace levels of benzene may be found in cigarette smoke, gasoline, glues, adhesives, cleaning products, and paint strippers.
“Valisure’s research identifying benzene contamination in multiple over-the-counter sunscreen products is an extremely important discovery for several reasons. First, it warns people practicing sun protection and skin care that some, but not all, sunscreens have potentially hazardous benzene contamination. Second, it is important for people, especially heading into the summer months, to understand that many sunscreen products tested by Valisure did not have benzene contamination, and those products are presumably safe and should continue to be used, along with appropriate hats and sun-protective clothing, to mitigate skin cancer risk,” according to Dr. Bunick.
“I believe it is critical that regulatory agencies address benzene contamination in sunscreens, and all topical medications at the manufacturing and final product level, so that all individuals feel safe using sunscreen products.”
Valisure’s independent chemical testing has identified several serious drug quality issues, which have resulted in global recalls of ranitidine, a once common antacid medication, metformin, a top diabetes drug with roughly 90 million prescriptions written per year in the United States, and hand sanitizer products, marking the first broadly FDA announced drug product recalls for benzene.
“These findings of benzene in sunscreens and after-sun care products build upon our substantiated findings of benzene in hand sanitizers that have already led to national recalls,” stated David Light. “It is unfortunately apparent that benzene contamination is a broad and very concerning issue in the American consumer product supply chain and underscores the critical need for independent testing. It is imperative for FDA to expeditiously address current regulatory gaps regarding benzene in both drug and cosmetic products, and we urge FDA and manufacturers to quickly investigate and remove contaminated products from the market.”
Complete Valisure FDA Citizen Petition Documents:
Full Citizen Petition – Contains lists of products where benzene was detected.
Attachment A – Contains list of products where benzene was not detected.
Attachment B – Resolution from the American College of Cardiology regarding independent testing.
Please read about Responsible Disposal of Potentially Contaminated Products.
aSunscreen products are often available in dozens of formulations from numerous companies, and it is estimated by FDA that over 11,000 sunscreen products are on market in the United States. To further assess the pervasiveness of the presence of benzene in sunscreen and after-sun care products on the U.S. market, Valisure is conducting a crowdsourcing study by inviting participants to send in their sunscreen and after-sun care products for testing. For more information and to participate in this study, please click here.
bThe views and opinions contained at ConsumerLab.com and its related reports on sun care products are that of ConsumerLab.com, LLC and do not reflect the opinions and beliefs of Valisure, LLC, its members, or Dr. Christopher Bunick.
About Valisure: Valisure’s core mission is to independently check the chemical composition of medications and healthcare products before they reach consumers and deliver that transparency throughout the supply chain as a differentiating partner for quality. In response to rising concerns about counterfeits, generics, and overseas manufacturing, Valisure’s team of Harvard- and Yale-trained scientists developed proprietary analytical technologies to screen products, identify critical issues, and offer certification to help distinguish quality stakeholders and products. Valisure has ISO 17025 accreditation and is DEA and FDA registered.